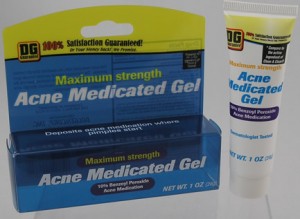
Benzoyl Peroxide Acne Cream 10% Contained Burkholderia Cepacia Bacteria
Benzoyl Peroxide Acne Cream 10% marked as:
DG Maximum Strength Acne Medicated Gel
Kroger Acne Gel 10% Benzoyl Peroxide Acne Medication
Equate: Medicated Acne Gel
Audience: Consumers, dermatologists, pharmacists, other healthcare professionals
CSI USA Inc. and FDA informed consumers and healthcare professionals of a nationwide recall of all lots of 1 ounce tubes of 10% Benzoyl Peroxide Acne Cream. The products were recalled because samples of the products were found to contain bacteria, Burkholderia Cepacia, formerly known as Pseudomonas Cepacia. There may be an increased health risk of infections for individuals with cuts, scrapes, rashes or other compromised skin conditions; or those with weakened or suppressed immune systems. Consumers should discontinue using the product and should return it to the place of purchase.