FDA warns against body-building products
The Food and Drug Administration warned the public Tuesday not to use body-building products marketed as containing steroids or steroid-like substances.
FDA said many of the products are sold as dietary substances, but are actually unapproved and misbranded drugs.
“Products marketed for body building and claiming to contain steroids or steroid-like substances are illegal and potentially quite dangerous,” said the agency’s commissioner, Dr. Margaret Hamburg, “The FDA is taking enforcement action today to protect the public.”
The agency sent a warning letter to American Cellular Laboratories Inc., saying it markets and distributes products that are labeled dietary supplements, but that violate the Federal Food, Drug and Cosmetic Act because they do not meet the definition of dietary supplement. To be a dietary supplement, the product has to contain one or more dietary ingredients, such as vitamins, minerals, amino acids, or herbs.
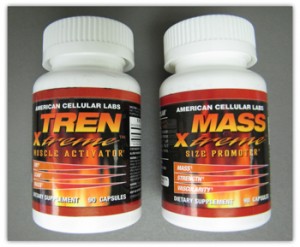
The affected products are; TREN-Xtreme, MASS Xtreme, ESTRO Xtreme, AH-89-Xtreme, HMG Xtreme, MMA-3 Xtreme, VNS-9 Xtreme, and TT-40-Xtreme.
They are sold as muscle stimulants for body builders who want to add muscle, lose weight and retain muscle mass. Sold mostly at gyms, by mail order and over the Internet, the products claim to have steroid-like ingredients but actually contain synthetic steroids, the agency said.
Another 15 events were attributed to this type of product in general, it said.
“Due to the potentially serious health risks associated with using these types of products, the FDA recommends that consumers immediately stop using all body-building products that claim to contain steroids or steroid-like substances,” the agency said in a public health advisory.
FDA recommends that anyone taking the products stop immediately and see a doctor if they experience various side effects.
American Cellular Laboratories has been given 15 business days to respond in writing to the warning letter, outlining the corrective steps they plan to take.